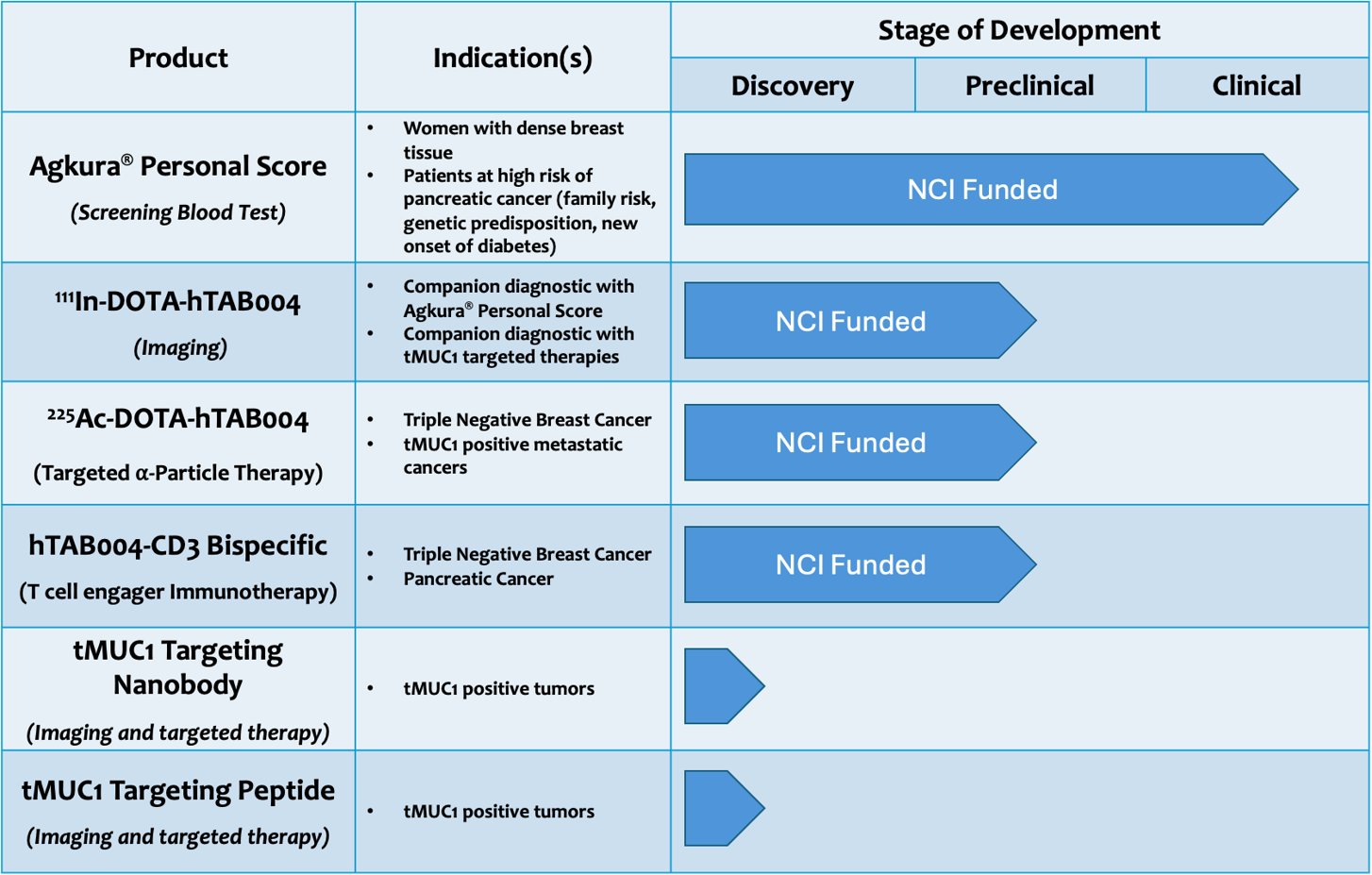
The first targeting agent we have developed is a tMUC1 specific antibody, TAB004. This targeting agent has been fully humanized (hTAB004) and has been used to develop four products, all of which have received funding from the US National Cancer Institute following rigorous scientific reviews:
- Agkura® Personal Score: A simple blood test to detect the growth or progression of epithelial cancers (Validated for breast and pancreatic cancers in clinical studies1,2)
- Radiolabeled hTAB004 to image tumors in preclinical studies (Validated for breast3 and pancreatic cancer)
- Radiolabeled hTAB004 as a therapeutic agent in preclinical studies (Validated for triple negative breast cancer3)
- Engineered hTAB004 T-cell engaging bi-specific antibody in combination with chemotherapy in preclinical studies (Validated for pancreatic cancer). This product was developed based on preclinical results using T cells engineered with TA004 scFv to treat triple negative and pancreatic cancer4,5,6
REFERENCES
- Roy LD, Dillon LM, Zhou R, Moore LJ, Livasy C, El-Khoury JM, Puri R, Mukherjee P: A tumor specific antibody to aid breast cancer screening in women with dense breast tissue. Genes & Cancer 2017, 8(3-4):536-549 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5489651/
- Salmon JSe: Phase II study of regorafenib (Reg) in patients with previously treated advanced pancreatic cancer (APC). JCO 2017, 35(15 suppl.). https://ascopubs.org/doi/10.1200/JCO.2017.35.15_suppl.e15751
- Kelly VJ, Wu ST, Gottumukkala V, Coelho R, Palmer K, Nair S, Erick T, Puri R, Ilovich O, Mukherjee P: Preclinical evaluation of an (111)In/(225)Ac theranostic targeting transformed MUC1 for triple negative breast cancer. Theranostics 2020, 10(15):6946-6958 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7295045/
- Zhou R, Yazdanifar M, Roy LD, Whilding LM, Gavrill A, Maher J, Mukherjee P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Frontiers in immunology, 2019 10: 1149 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6543840/
- Yazdanifar M, Zhou R, Grover P, Williams C, Bose M, Moore LJ, Wu S-t, Chi R, Maher J, Dreau D. Overcoming Immunological Resistance Enhances the Efficacy of a Novel anti-tMUC1 CAR T Cell Treatment Against Pancreatic Ductal Adenocarcinoma. 2019, bioRxiv: 642934 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6770201/
- Ru Zhou, Shu-ta Wu, Mahboubeh Yazdanifar, Chandra Williams, Alexa Sanders, Cory Brouwer, John Maher, and Pinku Mukherjee. Mucin-1–Targeted Chimeric Antigen Receptor T Cells Are Effective and Safe in Controlling Solid Tumors in Immunocompetent Host, Journal of Immunotherapy ():10.1097/CJI. 0000000000000505, January 25, 2024. https://journals.lww.com/immunotherapy-journal/fulltext/9900/mucin_1_targeted_chimeric_antigen_receptor_t_cells.83.aspx

